[ad_1]
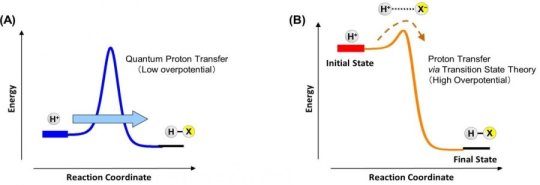
NIMS and Hokkaido University jointly discovered that proton transfer in electrochemical reactions is governed by the quantum tunneling effect (QTE) under the specific conditions. In addition, they made a first ever observation of the transition between the quantum and classical regimes in electrochemical proton transfer by controlling potential. These results indicated the involvement of QTE in electrochemical proton transfer, a subject of a long-lasting debate, and may accelerate basic research leading to the development of highly efficient electrochemical energy conversion systems based on quantum mechanics.
Many of the state-of-the-art electronic devices and technologies that have realized present modern lives were established based on the fundamental principles of quantum mechanics. Quantum effects in electrochemical reactions in fuel cells and energy devices are, however, not well understood due to the complex movement of electrons and protons driven by electrochemical reaction processes on the surfaces of electrodes. As the result, application of quantum effects in electrochemical energy conversion is not as successful as the fields of electronics and spintronics, which surface and interfacial phenomena are equally critical in all of these fields. Assuming that electrochemical reactions are closely associated with quantum effects, it may be feasible to design highly efficient energy conversion mechanisms based on these effects: including QTE, and devices that take advantage of such mechanisms.
In this study, the NIMS-led research team focused on oxygen reduction reaction (ORR) mechanisms — the key reaction in fuel cells — using deuterium, an isotope of hydrogen having a different mass. As a result, the team confirmed proton tunneling through activation barriers within a small overpotential range. Furthermore, the team found that an increase in overpotential leads to electrochemical reaction pathways to change to proton transfer based on the semiclassical theory. Thus, this research team discovered the novel physical processes: the transition between the quantum and classical regimes in electrochemical reactions.
This research shows the involvement of QTE in proton transfer during the basic energy conversion processes. This discovery may facilitate investigations of microscopic mechanisms of electrochemical reactions which are not understood in detail. It may also stimulate the development of highly efficient electrochemical energy conversion technology with a working principle based on quantum mechanics, capable of operating beyond the classical regime.
Story Source:
Materials provided by National Institute for Materials Science, Japan. Note: Content may be edited for style and length.
[ad_2]