Scientists from the JU Faculty of Biochemistry, Biophysics and Biotechnology have recently published a paper in the prestigious journal Proceedings of the National Academy of Sciences of the United States of America (PNAS). By studying mitochondrial disease mutations, they have shown how haem-proteins change their properties in order to effectively transfer electrons.
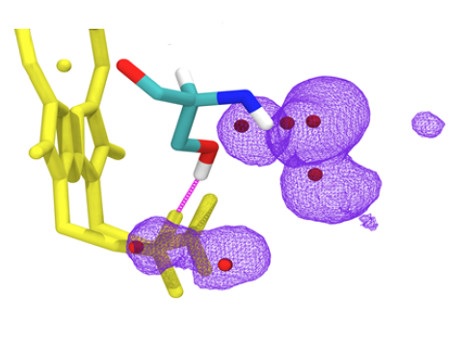
Cells contain complicated protein machineries, aimed at transforming energy in order to maintain vital functions. These processes are based on electron transfer, which takes place with the help of various factors, including haem groups anchored in proteins. In order for the transfer to be effective, the haem groups must posses certain physical and chemical properties. It turns out that even slight structural changes may significantly impact these properties, although the specific mechanism of such changes is not always known. This is the case with a number of mitochondrial diseases, where changes caused by a single protein mutation can manifest in such symptoms as exercise intolerance.
Researchers from the Department of Molecular Biophysics at the Jagiellonian University Faculty of Biochemistry, Biophysics and Biotechnology have taken a closer look on one of such mitochondrial mutations. Applying advanced spectroscopy techniques using electron paramagnetic resonance measurements in very low temperatures they revealed untypical properties of haem in the mutated protein. The obtained results were supplemented with molecular dynamics simulations (performed in collaboration with researchers from the University of Helsinki), which indicated that in order to maintain effective electron transfer in the protein, it is crucial to stabilise the specific element of its structure: hydrogen bonding to haem. Based on these observations a rule has been suggested according to which the properties of haem can be modulated within a specific range. This rule probably has a universal character and also applies to other haem proteins. Hence, it can be useful in designing artificial proteins and active systems containing haem, applied in nanotechnology and other areas.
The PNAS article entitled “Hydrogen bonding rearrangement by a mitochondrial disease mutation in cytochrome bc1 perturbs heme bH redox potential and spin state” has been authored by JU researchers: Dr Patryk Kuleta, Dr hab. Marcin Sarewicz, Iwona Ekiert, Dr Robert Ekiert, and Prof. Artur Osyczka as well as scientists from the University of Helsinki: Jonathan Lasham and Vivek Sharma. The project was funded by the Foundation for Polish Science (Homing Plus grant for Dr. Robert Ekiert) and the National Science Centre (Maestro grant for Prof. Artur Osyczka).