Photo: The bromide ion (Br-) in the solution can be easily exchanged with the iodide ion (I-) inside the crystal. The movement of the halide ions inside the crystal is instigated…
view more
Credit Image: Tachikawa Takashi
Hybrid organic-inorganic perovskites (*1) have received much attention as potential next generation solar cells and as materials for light-emitting devices.
Kobe University’s Associate Professor TACHIKAWA Takashi (of the Molecular Photoscience Research Center) and Dr. KARIMATA Izuru (previously a graduate student engaged in research at the Graduate School of Science) have succeeded in completely substituting the halide ions of perovskite nanocrystals while maintaining their morphology and light-emitting efficiency.
Furthermore, by using techniques such as single-particle photoluminescence imaging, the researchers were able to understand the momentary changes in light emission and the crystal structure, which in turn enabled them to develop a principle for controlling ion composition.
It is expected that these research results will contribute towards enabling the synthesis of perovskites of varying compositions and advancing the development of devices which utilize them. In addition, it is hoped that the flexibility of perovskite structures can be harnessed, allowing for them to be applied to devices and the creation of new functional materials.
These findings were published in the German academic journal ‘Angewandte Chemie International Edition‘ on October 19, 2020.
Research Background
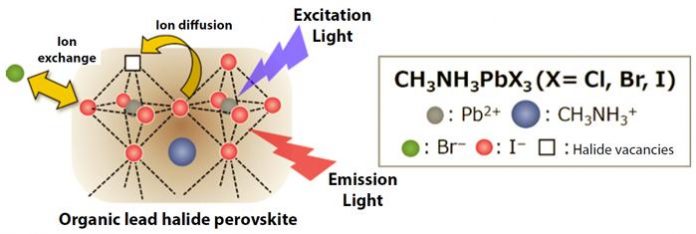
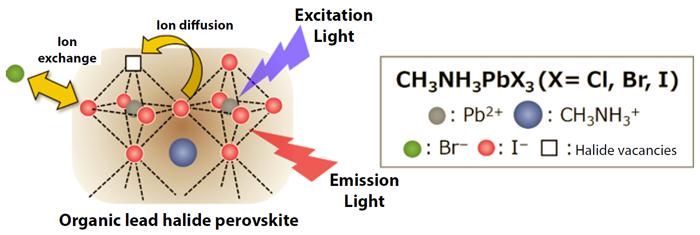
Hybrid organic-inorganic perovskites, such as organic lead halide perovskites (for example, CH3NH3PbX3 (X = Cl, Br, I)), have been receiving worldwide attention as a promising material for highly efficient solar cells (Figure 1). Furthermore, the color of the light that they emit can be controlled by altering the type and composition of the halide ions. Consequently, it is hoped that hybrid organic-inorganic perovskites can be applied to light-emitting devices such as displays and lasers.
However, the halide ions inside the crystals are known to move around even at room temperature, and this high flexibility causes issues such as reductions in both synthesis reproducibility and device durability.
Research Methodology
In this study, the researchers used a custom-made flow reactor (*3) to precisely control the exchange reaction between the CH3NH3PbI3 nanocrystals and Br– ions in solution. This enabled them to successfully convert the nanocrystals into CH3NH3PbBr3 nanocrystals while maintaining their morphology and light-emitting efficiency (Figure 2).
It is important to know what kind of reaction will occur inside the crystals in order to develop synthesis techniques. To understand this, the researchers used a fluorescence microscope to observe how each individual nanocrystal was reacting. From this observation, they understood that once the red light emitted by the CH3NH3PbI3 had completely disappeared, the green light originating from the CH3NH3PbBr3 was suddenly generated after an interval of 10s to 100s of seconds (upper portion of Figure 2). Based on the results of structural analysis using an x-ray beam, it was revealed that Br– ions replaced I– ions inside the crystal structure while a bromide-rich layer formed on the surface. Afterwards, the bromide on the surface layer gradually moved into the inner regions.
It is believed that the red light emissions became unobservable because the inner regions of the crystal structure were partially disordered during the ion transition, which led to the loss of energy necessary for light emission (bottom of Figure 2). Subsequently, CH3NH3PbBr3 crystal nuclei formed inside the nanocrystal particle and a cooperative transition to the green light generating state occurred.
From these results, it can be said that temporally separating the crystal structure transitions and the subsequent restructuring (that occurs on a nanometer scale) is one of the keys to the successful, precise synthesis of organic lead halide perovskites.
Further Developments
The structural transformation process observed in perovskite nanocrystals in this study is thought to be related to all modes of nanomaterial synthesis that are based on ion exchange, therefore future research could hopefully illuminate the underlying mechanism. Although researchers have a negative impression of organic halide perovskites’ flexibility, it is hoped that this characteristic could be exploited and applied to the development of new materials and devices that can react to the environment and external stimuli.
###
Acknowledgements
This research was supported by the following Japan Society for the Promotion of Science KAKENHI grants: Grant-in-Aid for Scientific Research B (JP18H01944) and Grant-in-Aid for Scientific Research on Innovative Areas (JP18H04517 and JP20H04673).
Glossary
1. Hybrid organic-inorganic perovskite:
A perovskite-type compound consisting of both organic and inorganic ions. A typical organic lead halide perovskite consists of organic ions, halide ions and lead ions. Normally, perovskites such as calcium titanate (CaTiO3) are compounds with an ABO3 structure (A are trivalent metal ions and B are tetravalent metal ions).
2. Nanocrystal:
A nanometer-scale microcrystal. One nanometer (10-9m) is equal to a billionth of 1m. This study used crystals of approximately 90 nanometers.
3. Flow Reactor:
An apparatus that enables reactions to be conducted with multiple flowing solutions. In this study, the nanocrystals were immobilized on a glass substrate. As a solution containing an iodide ion flowed over the glass substrate, the emitted light resulting from the ion exchange reaction was observed under a microscope.
Journal Information
Title:
“In Situ Exploration of the Structural Transition during Morphology- and Efficiency-Conserving Halide Exchange on a Single Perovskite Nanocrystal”
DOI: 10.1002/anie.202013386
Authors:
Izuru Karimata, Takashi Tachikawa
Journal:
Angewandte Chemie International Edition
TDnews (tunisiesoir.com)