[ad_1]
Plastic surgery to treat large cutaneous ulcers, including those seen in people with severe burns, bedsores or chronic diseases such as diabetes, may someday be a thing of the past. Scientists at the Salk Institute have developed a technique to directly convert the cells in an open wound into new skin cells. The approach relies on reprogramming the cells to a stem-cell-like state and could be useful for healing skin damage, countering the effects of aging and helping us to better understand skin cancer.
“Our observations constitute an initial proof of principle for in vivo regeneration of an entire three-dimensional tissue like the skin, not just individual cell types as previously shown,” says Salk Professor Juan Carlos Izpisua Belmonte, holder of the Roger Guillemin Chair and senior author of the new paper, published in the journal Nature on September 5, 2018. “This knowledge might not only be useful for enhancing skin repair but could also serve to guide in vivo regenerative strategies in other human pathological situations, as well as during aging, in which tissue repair is impaired.”
Cutaneous ulcers — wounds that can extend through multiple layers of the skin — are typically treated surgically, by transplanting existing skin to cover the wound. However, when the ulcer is especially large, it can be difficult for surgeons to graft enough skin. In these cases, researchers are able to isolate skin stem cells from a patient, grow them in the lab and transplant them back into the patient. However, such a procedure requires an extensive amount of time, which may put the patient’s life at risk and is sometimes not effective.
Izpisua Belmonte and Salk Research Associate Masakazu Kurita, who has a background in plastic surgery, knew that a critical step in wound recovery was the migration — or transplantation — of basal keratinocytes into wounds. These stem-cell-like cells act as precursors to the different types of skin cells. But large, severe wounds that have lost multiple layers of skin no longer have any basal keratinocytes. And even as these wounds heal, the cells multiplying in the area are mainly involved in wound closure and inflammation, rather than rebuilding healthy skin.
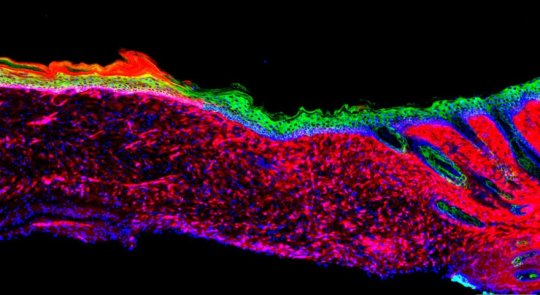
Izpisua Belmonte and Kurita wanted to directly convert these other cells into basal keratinocytes — without ever taking them out of the body. “We set out to make skin where there was no skin to start with,” says Kurita.
The researchers first compared the levels of different proteins of the two cell types (inflammation and keratinocytes) to get a sense of what they’d need to change to reprogram the cells’ identities. They pinpointed 55 “reprogramming factors” (proteins and RNA molecules) that were potentially involved in defining the distinct identity of the basal keratinocytes. Then, through trial and error and further experiments on each potential reprogramming factor, they narrowed the list down to four factors that could mediate the conversion to basal keratinocytes.
When the team topically treated skin ulcers on mice with the four factors, the ulcers grew healthy skin (known as epithelia) within 18 days. Over time, the epithelia expanded and connected to the surrounding skin, even in large ulcers. At three and six months later, the generated cells behaved like healthy skin cells in a number of molecular, genetic and cellular tests.
The researchers are planning more studies to optimize the technique and begin testing it in additional ulcer models.
“Before going to the clinic, we have to do more studies on the long-term safety of our approach and enhance the efficiency as much as possible,” says Kurita.
In addition to Kurita and Izpisua Belmonte, authors of the new paper were Toshikazu Araoka, Tomoaki Hishida, David D. O’Keefe, Yuta Takahashi, Akihisa Sakamoto, Masahiro Sakurai, Keiichiro Suzuki, Jun Wu, Mako Yamamoto, Reyna Hernandez-Benitez, Alejandro Ocampo, Pradeep Reddy and Maxim Nikolaievich Shokhirev of the Salk Institute; Pierre Magistretti of King Abdullah University of Science and Technology; Estrella Núñez Delicado of Universidad Católica San Antonio de Murcia; and Hitomi Eto and Kiyonori Harii of Kyorin University School of Medicine.
The work and the researchers involved were supported by grants from Japan’s Ministry of Education, Culture, Sports, Science and Technology (MEXT); Kyorin University; the Japan Society for the Promotion of Science; the Uehara Memorial Foundation; the National Cancer Institute; the G. Harold and Leila Y. Mathers Charitable Foundation; The Leona M. and Harry B. Helmsley Charitable Trust; The Moxie Foundation; The Evergreen Foundation; Fundacion Dr. Pedro Guillen; and Universidad Católica San Antonio de Murcia.
Story Source:
Materials provided by Salk Institute. Note: Content may be edited for style and length.
[ad_2]