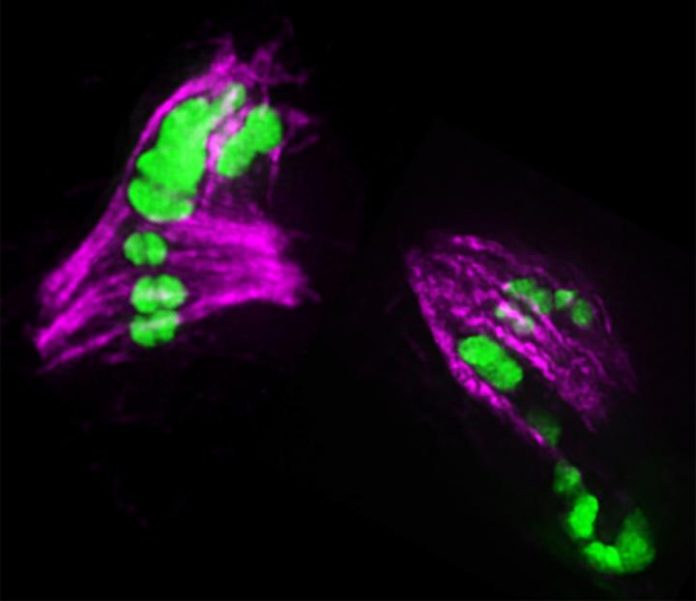
Photo: Fluorescent image shows chromosomes (green) segregating in two developing eggs. In each egg, one chromosome (the largest green one) has too many crossovers and is having problems segregating. The image…
view more
Credit Image: Image courtesy of Diana Libuda
EUGENE, Ore. – Oct. 8, 2020 – The exchange of DNA between chromosomes during the early formation of sperm and egg cells normally is limited to assure fertility.
But when there are too many of these genetic exchanges, called crossover events, the segregation of chromosomes into eggs is flawed, biologists have learned in a project done across three labs at the University of Oregon and Northwestern University.
In a paper published online Sept. 4 in the journal PLOS Genetics, researchers documented how the disruptions, as seen in basic research with microscopic roundworms (Caenorhabditis elegans), lead to a range of meiotic defects as the chromosomes are subjected to improper spindle forces.
Inaccurate chromosome segregation in humans is associated with Down syndrome and miscarriages. Such segregation defects as seen in the research can result in increased infertility, said UO biologist Diana E. Libuda, the study’s principal investigator.
“Over the past century, research has focused on making sure enough crossovers are made during sperm and egg development,” said Libuda, a professor in the UO’s Department of Biology and Institute of Molecular Biology. “It was known that developing sperm and eggs had ways to make sure that not too many crossovers are made, but it was unclear why.”
The research team identified two mechanisms that help counteract defects triggered by excess crossover activity in developing eggs and, thus, assist the coordination of the process that helps assure genomic integrity in new generations.
Libuda had reported in the Oct. 9, 2013, issue of Nature the discovery of a mechanism that inhibits the overproduction of crossovers in roundworms. However, Libuda said, it was not possible at that time to study the downstream effects in cases where too many crossovers did occur. Since then, her lab developed a way to generate extra crossovers on a single chromosome.
That ability led to a National Institutes of Health-funded collaboration with Sadie Wignall of Northwestern University, an expert on high-resolution imaging of structures involved in segregation of chromosomes into developing eggs. What Wignall found led Libuda back to Bruce Bowerman’s UO lab to take a look at chromosome segregation in live developing eggs.
“Overall, it was a great joining of scientific strengths to take a multipronged approach to answer this important question,” Libuda said.
The research provides fundamental insights that can guide research in other organisms to better understand the mechanisms and, eventually, lead to potential clinical applications.
“The same proteins that we are studying in C. elegans are also in humans,” Libuda said. “In fact, most proteins required for fertility are used across organisms that include yeast, fruit flies, nematodes, zebrafish, mice and humans. Research using these microscopic worms has been shown in numerous contexts to have relevance in human health. “
###
Co-authors with Libuda, Bowerman and Wignall on the paper were: Jeremy A. Hollis, a technician in Wignall’s lab; former UO biology undergraduate student Marissa L. Glover, now a doctoral student at the University of California, Santa Cruz; Aleesa J. Schlientz, who earned a doctorate from the UO this year; and Cori K. Cahoon, a postdoctoral researcher working in Libuda’s lab under a fellowship from the Jane Coffin Childs Memorial Fund for Medical Research.
Media Contact: Jim Barlow, director of science and research communications, 541-346-3481, [email protected]
Related Links:
About Diana Libuda: http://molbio.
Libuda Lab: http://www.
UO Department of Biology: https:/
UO Institute of Molecular Biology: http://molbio.
About Bruce Bowerman: http://molbio.
Wignall Lab: https:/
TDnews (tunisiesoir.com)