[ad_1]
Researchers from Tokyo Metropolitan University and the FIRC Institute of Molecular Oncology (IFOM) in Italy have succeeded in depleting AND-1, a key protein for DNA replication, by using a recently developed conditional protein degradation system. Consequently, they were able to gain unprecedented access to the mechanism behind how AND-1 works during DNA replication and cell proliferation in vertebrate cells, demonstrating that AND-1 has two different functions during DNA replication mediated by different domains of AND-1.
DNA is often referred to as the “blueprint of life”; in order for living organisms to function, it is vital that all cells share the same blueprint. This is made possible by the process of DNA replication, where the DNA is accurately copied and distributed before the cell multiplies. Replication underpins all biological inheritance, and is supported by a whole range of biochemical pathways designed to ensure that it occurs without error and at the right speed. Failure to do so may have catastrophic consequences, including cancer: understanding the specific mechanisms behind this highly complex procedure is of the utmost importance.
The AND-1/Ctf4 protein is a key player in DNA replication, and is found in a vast range of living organisms, from fungi to vertebrates. Ctf4/AND-1 is essential in some organisms, but whether it is an essential gene for cell proliferation in vertebrates has not been shown experimentally. Moreover, how loss of AND-1 affects cell proliferation is not known.
In order to address this question, a team led by Dr. Dana Branzei from IFOM and Prof. Kouji Hirota from Tokyo Metropolitan University combined the use of two unique systems, the DT40 cell, a type of avian cell that is particularly suited to genetic engineering, and the auxin-inducible degron (AID) system, a means to realize selective depletion of a target protein. With these, they successfully established the and-1-aid cell line, in which a modified version of the AND-1 protein is degraded in a few hours after adding auxin, a type of plant hormone. This cell line enabled them to analyze the acute consequence of AND-1 loss, giving unprecedented insight into the role it played.
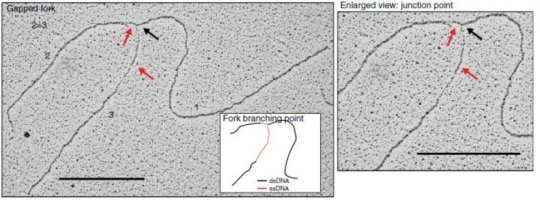
When done correctly, DNA replication should result in the formation of new double-stranded DNA helices. Authors used transmission electron microscopy (TEM) to visualize DNA replication intermediates and observed newly synthesized DNA having abnormally long single stranded DNA at the fork branching point in the absence of AND-1. They hypothesized that this was due to a DNA cleaving enzyme, a nuclease, disrupting the process of strands being disassembled. On further addition of a compound that suppresses the action of a particular nuclease, MRE11, they were able to successfully revert the abnormal replication fork phenotype and recover cell division, explicitly demonstrating the key role played by AND-1 in preventing nascent DNA cleavage by the nuclease during replication. Further analysis revealed that a specific part of the protein called WD40 repeats was responsible for preventing the accumulation of damage to the strand.
Further to these breakthrough findings, the study highlights the successful combination of cutting-edge techniques to realize conditional inactivation of specific proteins; the new cells were in fact developed over a single month. This leaves the exciting prospect of the method being applied to study other genes and processes which are otherwise difficult to target, leading to new insights into how cells work.
This work was supported by the Italian Association for Cancer Research (IG 14171 and IG 18976), the European Research Council (Starting Grant 242928 and Consolidator Grant 682190) grants and a JSPS KAKENHI (JP16H02957). The study has been published online in the journal Nature Communications.
Story Source:
Materials provided by Tokyo Metropolitan University. Note: Content may be edited for style and length.
[ad_2]