[ad_1]
Scientists have long sought to develop drug therapies that can more precisely diagnose, target and effectively treat life-threatening illness such as cancer, cardiovascular and autoimmune diseases. One promising approach is the design of morphable nanomaterials that can circulate through the body and provide diagnostic information or release precisely targeted drugs in response to disease-marker enzymes. Thanks to a newly published paper from researchers at the Advanced Science Research Center (ASRC) at The Graduate Center of The City University of New York, Brooklyn College, and Hunter College, scientists now have design guidance that could rapidly advance development of such nanomaterials.
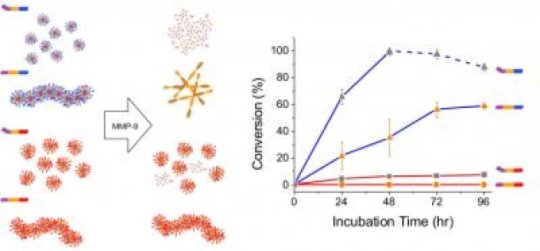
In the paper, which appears online in the journal ACS Nano, researchers detail broadly applicable findings from their work to characterize a nanomaterial that can predictably, specifically and safely respond when it senses overexpression of the enzyme matrix metalloproteinase-9 (MMP-9). MMP-9 helps the body breakdown unneeded extracellular materials, but when levels are too high, it plays a role in the development of cancer and several other diseases.
“Right now, there are no clear rules on how to optimize the nanomaterials to be responsive to MMP-9 in predictable ways,” said Jiye Son, the study’s lead author and a Graduate Center Ph.D. student working in one of the ASRC Nanoscience Initiative labs. “Our work outlines an approach using short peptides to create enzyme-responsive nanostructures that can be customized to take on specific therapeutic actions, like only targeting tumor cells and turning on drug release in close proximity of these cells.”
Methodology
Researchers designed a modular peptide that spontaneously assembles into nanostructures, and predictably and reliably morphs or breaks down into amino acids when they come in contact with the MMP-9 enzyme. The designed components include a charged segment of the nanostructure to facilitate its sensing and engagement with the enzyme; a cleavable segment of the structure so that it can lock onto the enzyme and determine how to respond; and a hydrophobic segment of the structure to facilitate self-assembly of the therapeutic response.
Significance
“This work is a critical step toward creating new smart-drug delivery vehicles and diagnostic methods with precisely tunable properties that could change the face of disease treatment and management,” said ASRC Nanoscience Initiative Director Rein Ulijn, whose lab is leading the work. “While we specifically focused on creating nanomaterials that could sense and respond to MMP-9, the components of our design guidance can facilitate development of nanomaterials that sense and respond to other cellular stimuli.”
Among other advances, the research team’s work builds on their previous findings, which showed that amino acid peptides can encapsulate and transform into fibrous drug depots upon interaction with MMP-9. The group is collaborating with scientists at Memorial Sloan Kettering and Brooklyn College to use their findings to create a novel cancer therapy.
Story Source:
Materials provided by Advanced Science Research Center, GC/CUNY. Note: Content may be edited for style and length.
[ad_2]