[ad_1]
HIV-1 replicates in ninja-like ways. The virus slips through the membrane of vital white blood cells. Inside, HIV-1 copies its genes and scavenges parts to build a protective bubble for its copies. Scientists don’t understand many of the details of how HIV-1 can fool our immune system cells so effectively. The virus infects 1.2 million people in the U.S. and 37 million people worldwide in 2018. Supercomputers helped model a key building block in the HIV-1 protective capsid, which could lead to strategies for potential therapeutic intervention in HIV-1 replication.
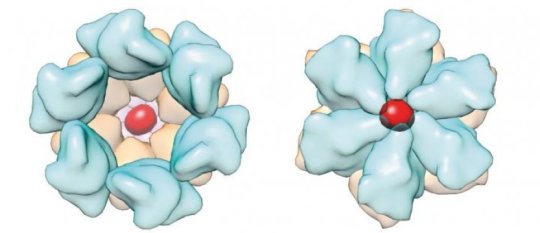
Scientists found the naturally-occurring compound inositol hexakisphosphate (IP6) promotes both assembly and maturation of HIV-1. “We discovered, in collaboration with other researchers, that HIV uses this small molecule to complete its function,” said Juan R. Perilla, Department of Chemistry and Biochemistry, University of Delaware. “This is a molecule that’s extremely available in human cells and in other mammalian cells. HIV has evolved to make use of these small molecules present in our cells to essentially be infectious.” Perilla co-authored the study in the journal Nature in August 2018.
Perilla ran simulations of inositol phosphate interactions with HIV structural proteins CA-CTD-SP1 using NAMD through allocations on XSEDE, the Extreme Science and Engineering Environment, funded by the National Science Foundation. “XSEDE provides a unique framework which allows us to use computational resources that are tailored to the needs of a particular scientific problem. In addition, we benefit from the HPC training opportunities provided by XSEDE which allows us to develop novel analysis tools,” Perilla said.
The allocation included time on the Anton2 system of the Pittsburgh Supercomputing Center to run atomistic simulations of bound IP6. “Anton2 enabled us to perform long-scale simulations to test the stability of the immature capsid assembly and IP6,” Perilla said.
Through XSEDE, the Stampede2 system at the Texas Advanced Computing Center ran NAMD simulations of the Inositol phosphates IP3, IP4, IP5 and their interactions with HIV proteins CA-CTD-SP1. “What Stampede2 allowed us to do is establish what the molecular interactions are between the HIV proteins and this small molecule and to test the hypothesis that it was stabilizing a particular part of the protein using molecular dynamics,” said Juan Perilla.
“I think Stampede2 is a great machine, and it’s extremely beneficial to the scientific community to have a resource like that available on a merit-based system. What I would like the public to know is that it’s important that these large-scale machines are available. They are not just a replacement of a small cluster. These machines really enable new science. If you didn’t have machines of this scale, you couldn’t do the kind of science that we do because our problems are larger than what you can have on a campus cluster. We really need to have the scale of these machines available to the scientific community to enable the kind of science that we do,” Perilla said.
Perilla described the increasing use of the ‘computational microscope,’ the combination of supercomputers with laboratory data. “With the computational microscope, you can see how things move. Many experimental techniques are just a snapshot. With the computational microscope, you can actually see how things are moving,” he said.
Supercomputer modeling of how building blocks of HIV-1 move in time made a difference in this study. “That discovery opens a door for development of new treatments. It’s a therapeutic target. Because of that, it makes it very appealing for drug development and therapeutic development,” Perilla said.
There remains much to be learned about HIV-1 behaves, said Perilla. “We’re basic scientists. NSF’s mission is to understand these systems as living organisms. The overall idea is that we want to understand the virus as a biological problem and ultimately this knowledge will be used to derive therapeutics,” Perilla said.
The study, “Inositol phosphates are assembly cofactors for HIV-1,” was published in the journal Nature on August 1, 2018. The study authors are Robert A. Dick and Volker M. Vogt of Cornell University; Kaneil K. Zadrozny, Jonathan M. Wagner, Barbie K. Ganser-Pornillos, and Owen Pornillos of the University of Virginia; Chaoyi Xu” and Juan R. Perilla of the University of Delaware; Florian K. M. Schur of the European Molecular Biology Laboratory and the Institute of Science and Technology Austria; Terri D. Lyddon, Marc C. Johnson, and Clifton L. Ricana of the University of Missouri. The National Institutes of Health funded the research. This work used the Extreme Science and Engineering Discovery Environment, which is supported by National Science Foundation grant number OCI-1053575.
[ad_2]