[ad_1]
Prions can infect both humans and animals, causing Creutzfeldt-Jakob disease (CJD) in humans, mad cow disease in cattle, and chronic wasting disease in elk and deer. The infectious, misfolded protein particles often go undetected as they destroy brain tissue, causing memory loss, mobility issues, and ultimately death. Preclinical detection of prions has proven difficult, but new research suggests skin samples hold early signs of prion disease that precede neurologic symptoms.
“Currently a definitive diagnosis of Creutzfeldt-Jakob disease is dependent on the examination of diseased brain tissue obtained at biopsy or autopsy. It has been impossible to detect at the early preclinical stage,” said Wenquan Zou, MD, PhD, associate professor of pathology at Case Western Reserve University School of Medicine.
In a ground-breaking study published in Nature Communications, Zou and an international team of researchers successfully used two methods to detect prions in skin samples collected from inoculated rodents. The study provides the first proof-of-concept evidence that readily accessible skin samples could be used to detect prion disease early — before clinical symptoms appear.
In the new study, Zou and colleagues successfully detected prions in rodent skin samples as early as two weeks post-infection. They also detected prions in the skin of uninoculated rodents that were housed alongside inoculated cage mates, demonstrating that prion transmission can occur between cohabitating rodents.
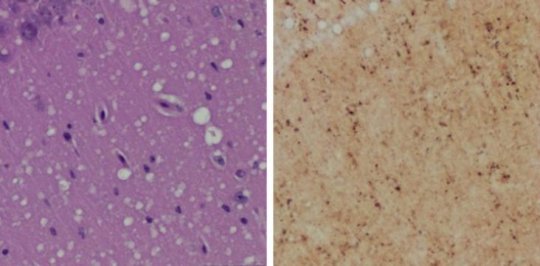
Prions were detected in skin samples from the inoculated rodents before they showed any clinical signs of prion disease. The researchers first inoculated the brains of hamsters and humanized transgenic mice with rodent or human prion samples, respectively. Then, they collected skin and brain samples at different time points, and used two different methods to detect disease-associated prion proteins in the tissues. In both hamsters and mice, the researchers detected prions in skin before they could be detected in brain tissue. The researchers concluded that skin prions could serve as a useful biomarker for preclinical diagnosis of prion diseases.
The study compared two highly-sensitive prion detection methods: RT-QuIC (real-time quaking-induced conversion) and sPMCA (serial protein misfolding cyclic amplification). “Both assays were able to efficiently amplify trace amounts of disease-associated prion protein found in the skin tissues of infected animals,” said the study’s first author Zerui Wang, MD, a PhD student from the First Hospital of Jilin University, China, working in the Zou laboratory. The tests use prions in tissue samples as a template and either normal brain tissue or synthetic prion protein as “building blocks” to dramatically amplify minute amounts of prions to detectable levels.
One of the methods, RT-QuIC, has been used to detect prion particles in symptomatic CJD patients. However, it normally requires invasive cerebrospinal fluid (CSF) sampling that may be contraindicated for certain patients. Additionally, “The CSF-based prion test results could be uncertain in some cases and not all CSF specimens from patients with prion disease are RT-QuIC positive,” said Qingzhong Kong, PhD, associate professor of pathology at Case Western Reserve University School of Medicine and co-corresponding author on the study. “Although skin samples may not replace CSF in routine RT-QuIC-based prion disease diagnosis, they may be helpful when prion disease is suspected but CSF is either unavailable or RT-QuIC-negative.”
The study results build upon previous work by Zou and colleagues showing that autopsy skin samples from human prion disease patients exhibit prion seeding and infectivity. The next step will be to develop and validate the skin prion tests for clinical use.
Said Zou, “Since the skin is readily accessible and skin biopsy is minimally invasive, detection of skin prions will be very useful for monitoring disease progression and assessing therapeutic efficacy during clinical trials or treatments when prion therapy becomes available in the future.”
Zou and Kong were recently awarded a $2.9 million grant from the National Institutes of Health to validate the test methods using human skin samples. They will determine if skin prions could serve as a diagnostic biomarker for CJD or a source of prion transmission.
The researchers believe the methods may also be adapted for diagnosis of other diseases involving misfolded proteins. “Sensitive, minimally invasive detection of various misfolded proteins in skin, such as tau in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease, could be highly valuable for disease diagnosis and monitoring of disease progression and efficacy of treatments,” Zou said. “It’s possible that the skin will ultimately serve as a mirror for us to monitor these misfolded proteins that accumulate and damage the brain in patients with these conditions.”
[ad_2]