[ad_1]
A new microscope system can image living tissue in real time and in molecular detail, without any chemicals or dyes, report researchers at the University of Illinois.
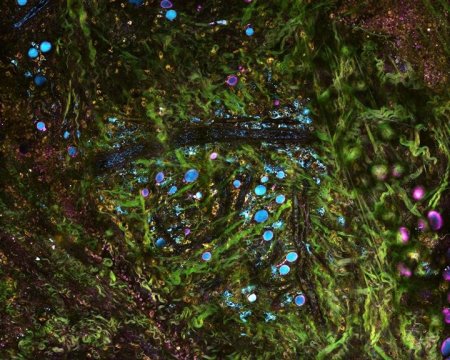
The system uses precisely tailored pulses of light to simultaneously image with multiple wavelengths. This enables the researchers to study concurrent processes within cells and tissue, and could give cancer researchers a new tool for tracking tumor progression and physicians new technology for tissue pathology and diagnostics.
The researchers detailed the technique, called simultaneous label-free autofluorescence multi-harmonic microscopy, in the journal Nature Communications.
“The way we have been removing, processing and staining tissue for diagnosing diseases has been practiced the same way for over a century,” said study leader Dr. Stephen Boppart, a professor of bioengineering and electrical and computer engineering at Illinois and a medical doctor. “With advances in microscopy techniques such as ours, we hope to change the way we detect, visualize and monitor diseases that will lead to better diagnosis, treatments and outcomes.”
SLAM microscopy differs from standard tissue pathology in several ways. First, it is used on living tissue, even inside a living being, giving it the potential to be used for clinical diagnosis or to guide surgery in the operating room. Second, it uses no dyes or chemicals, only light. Standard procedure involves removing a tissue sample and adding chemical stains — which can be a lengthy process — and the chemicals can disrupt the cells.
While other stain-free imaging techniques have been developed, they usually only visualize a subset of signals, measuring specific biological or metabolic signatures, said graduate student Sixian You, the first author of the paper. Meanwhile, SLAM microscopy simultaneously collects multiple contrasts from cells and tissues, capturing molecular-level details and dynamics such as metabolism.
In the most recent study, Boppart’s group looked at mammary tumors in rats, along with the surrounding tissue environment. Thanks to the simultaneous data, they were able to observe the range of dynamics as the tumors progressed and how different processes interacted.
“We know the tumor is there, but tumors support a whole ecosystem in the tissue,” You said. “They recruit healthy cells to support them. SLAM allows us to have a comprehensive picture of this ever-evolving tumor microenvironment at subcellular, molecular and metabolic levels in living animals and human tissue. Monitoring that process can help us better understand cancer progression, and in the future could lead to better diagnosis of how advanced a tumor is, and better therapeutic approaches aimed at halting the progression.”
The researchers saw that the cells near the tumor had differences in metabolism and morphology, indicating that the cells had been recruited by the cancer. In addition, they observed surrounding tissues creating infrastructure to support the tumor, such as collagen and blood vessels. They also saw communication between the tumor cells and the surrounding cells in the form of vesicles, tiny transport packages released by cells and absorbed by other cells.
“Previous work has shown that tumor cells release vesicles to lure the surrounding cells to support them,” You said. “Then the cells that have been recruited release their own vesicles to go back to the tumor. It’s a vicious cycle. It’s very different from the activity we see in our control samples with healthy tissue. The capability to see the dynamics of all these important players in authentic tumor environments can help shed light on this mysterious but critical process.”
Next, Boppart’s group is using SLAM microscopy to compare healthy tissue and cancer tissue in both rats and humans, focusing particularly on vesicle activity and how it relates to cancer aggressiveness. They are also working to make portable versions of the SLAM microscope that could be used clinically.
“There is a wealth of new data, information and biomarkers in the images we collect from fresh tissue that is still metabolically active, or in living organisms, where we can visualize the dynamics of individual cells and their collective behaviors,” said Boppart, who also is affiliated with the Carle Illinois College of Medicine and the Beckman Institute for Advanced Science and Technology at Illinois.
“These, we expect, will become new markers for diseases such as cancer, and our imaging technology will help detect these for disease screening, diagnostics and monitoring applications. We believe that this technology will open the possibility of complementing, or even replacing, standard histopathology processing, which is time- and labor-intensive and can only be done on removed, fixed, dead tissue,” he said.
The National Institutes of Health and a McGinnis Fellowship supported this work.
[ad_2]