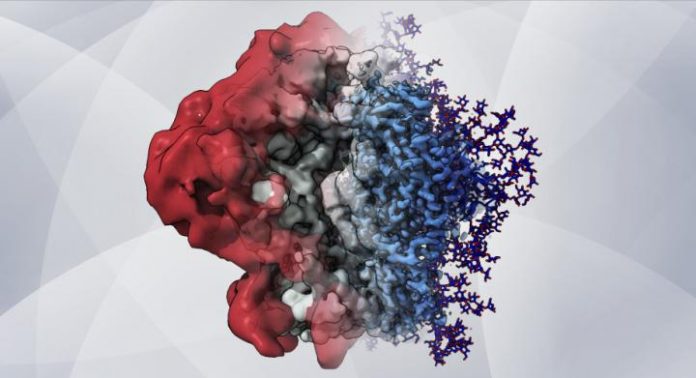
Photo: An artistic rendering–based on cryo-EM maps and computer simulations–shows how glycans create a shield that helps HIV hide from the immune system.
view more
Credit Image: Zachary Berndsen, PhD, Ward lab at Scripps Research
LA JOLLA, CA–Scientists from Scripps Research and Los Alamos National Laboratory have devised a method for mapping in unprecedented detail the thickets of slippery sugar molecules that help shield HIV from the immune system.
Mapping these shields will give researchers a more complete understanding of why antibodies react to some spots on the virus but not others, and may shape the design of new vaccines that target the most vulnerable and accessible sites on HIV and other viruses.
The sugar molecules, or “glycans,” are loose and stringy, and function as shields because they are difficult for antibodies to grip and block access to the protein surface. The shields form on the outermost spike proteins of HIV and many other viruses, including SARS-CoV-2, the coronavirus that causes COVID-19, because these viruses have evolved sites on their spike proteins where glycan molecules–normally abundant in cells–will automatically attach.
“We now have a way to capture the full structures of these constantly fluctuating glycan shields, which to a great extent determine where antibodies can and can’t bind to a virus such as HIV,” says the study’s lead author Zachary Berndsen, PhD, a postdoctoral research associate in the structural biology lab of Scripps Research Professor Andrew Ward, PhD.
The same wavy flexibility that makes these sugary molecules resistant to antibodies has made them impossible for researchers to capture with traditional atomic-scale imaging. In the new study, which appears in the Proceedings of the National Academy of Sciences, the scientists developed techniques that, for the first time, allow these elusive molecules to be mapped in great detail on the surface of the HIV spike protein, known as “Env.”
The Scripps Research team collaborated with the lab of Gnana Gnanakaran, PhD, staff scientist at Los Alamos National Laboratory, which is equipped with high-performance computing resources that enabled fresh approaches for modeling the glycans.
The researchers combined an atomic-scale imaging method called cryo-electron microscopy (cryo-EM) with sophisticated computer modeling and a molecule-identifying technique called site-specific mass spectrometry. Cryo-EM relies on averaging tens or hundreds of thousands of individual snapshots to create a clear image, thus highly flexible molecules like glycans will appear only as a blur, if they show up at all.
But by integrating cryo-EM with the other technologies, the researchers were able to recover this lost glycan signal and use it to map sites of vulnerability on the surface of Env.
“This is the first time that cryo-EM has been used along with computational modeling to describe the viral shield structure in atomic detail,” says Srirupa Chakraborty, PhD, co-lead author and post-doctoral researcher in the Gnanakaran lab at Los Alamos National Laboratory.
The new combined approach revealed the glycans’ structure and dynamic nature in extreme detail and helped the team better understand how these complex dynamics affect the features observed in the cryo-EM maps. From this wealth of information, the team observed that individual glycans do not just wiggle around randomly on the spike protein’s surface, as once was thought, but instead clump together in tufts and thickets.
“There are chunks of glycans that seem to move and interact together,” Berndsen says. “In between these glycan microdomains is where antibodies apparently have the opportunity to bind.”
Experimental HIV vaccines rely on modified, lab-made Env proteins to elicit antibody responses. In principle, these vaccines’ effectiveness depends in part on the positioning and extent of the shielding glycans on these lab-made viral proteins. Therefore, Berndsen and colleagues applied their method to map the glycans on a modified HIV Env protein, BG505 SOSIP.664, which is used in an HIV vaccine currently being evaluated in clinical trials.
“We found spots on the surface of this protein that normally would be covered with glycans but weren’t–and that may explain why antibody responses to that site have been noted in vaccination trials,” Berndsen says.
That finding, and others in the study, showed that Env’s glycan shield can vary depending on what type of cell is being used to produce it. In HIV’s infections of humans, the virus uses human immune cells as factories to replicate its proteins. But viral proteins used to make vaccines normally are produced in other types of mammalian cells.
In another surprise discovery, the team observed that when they used enzymes to slowly remove glycans from HIV Env, the entire protein began to fall apart. Berndsen and colleagues suspect that Env’s glycan shield, which has been considered merely a defense against antibodies, may also have a role in managing Env’s shape and stability, keeping it poised for infection.
The team expect that their new glycan-mapping methods will be particularly useful in the design and development of vaccines–and not only for HIV. Many of the techniques can be applied directly to other glycan-shielded viruses such as influenza viruses and coronaviruses, and can be extended to certain cancers in which glycans play a key role, the researchers say.
###
“Visualization of the HIV-1 Env glycan shield across scales” was authored by Zachary Berndsen, Srirupa Chakraborty, Xiaoning Wang, Christopher Cottrell, Jonathan Torres, Jolene Diedrich, John Yates III, James Paulson, Andrew Ward, Marit van Gils, Cesar Lopez and Gnana Gnanakaran.
Funding was provided by the National Institute of Allergy and Infectious Diseases (UM1 AI100663, UM1 AI144462, P41 GM103533), the National Institutes of Health (UM1 AI100645, UM1 AI144371), the Bill & Melinda Gates Foundation and the Center for Nonlinear Studies at the Los Alamos National Laboratory.