[ad_1]
A group of Japanese researchers has discovered that neural inflammation caused by our innate immune system plays an unexpectedly important role in stress-induced depression. This insight could potentially lead to the development of new antidepressants targeting innate immune molecules. The findings were published on July 20 in the online edition of Neuron.
The joint study was led by Professor Tomoyuki Furuyashiki and Assistant Professor Shiho Kitaoka (Kobe University Graduate School of Medicine) in collaboration with Project Professor Shuh Narumiya (Kyoto University Graduate School of Medicine).
Previous research had already hinted at the link between inflammation and depression: increased levels of inflammation-related cytokines in the blood of patients suffering from depression, activation of microglia (inflammation-related cells in the brain) in depressive patients, and a high percentage of depression outbreaks in patients suffering from chronic inflammatory disease. However, the exact relationship between depression and inflammation still contains many unknowns.
Psychological stress caused by social and environmental factors can trigger a variety of changes in both mind and body. Moderate levels of stress will provoke a defensive response, while extreme stress can lower our cognitive functions, cause depression and elevated anxiety, and is a risk factor for mental illnesses. The research team focused on repeated social defeat stress (a type of environmental stress) with the aim of clarifying the mechanism that causes an emotional response to repeated stress.
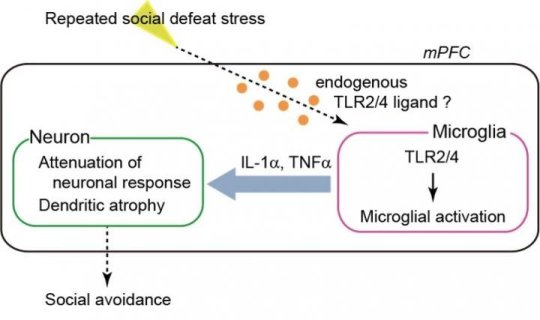
First, they looked at changes of gene expression in the brain caused by repeated social defeat stress and found that repeated stress increased a putative ligand for the innate immune receptors TLR2 and TLR4 (TLR2/4) in the brain. Their next step was to investigate the role of TLR2/4 in repeated stress using a mouse with the TLR2/4 genes deleted. They found that TLR2/4-deficient mice did not show social avoidance or extreme anxiety when exposed to repeated stress. Repeated stress usually triggers microglial activation in specific areas of the brain such as the medial prefrontal cortex, causing impaired response and atrophy of neurons, but these responses were not present in the TLR2/4-deficient mice.
The research team then developed a method to selectively block the expression of TLR2/4 in the microglia of specific areas of the brain. By blocking the expression of TLR2/4 in the microglia of the medial prefrontal cortex, they managed to suppress depressive behavior in response to repeated social defeat stress. They found that repeated stress induced the expression of inflammation-related cytokines IL-1? and TNF? in the microglia of the medial prefrontal cortex via TLR2/4. The depressive behavior was suppressed by treating the medial prefrontal cortex with neutralizing antibodies for the inflammation-related cytokines.
These results show that repeated social defeat stress activates microglia in the medial prefrontal cortex via the innate immune receptors TLR2/4. This triggers the expression of inflammation-related cytokines IL-1? and TNF?, leading to the atrophy and impaired response of neurons in the medial prefrontal cortex, and causing depressive behavior.
Professor Furuyashiki says: “These findings demonstrate the importance of neural inflammation caused by the innate immune system for stress-induced depression. This could lead to the development of new antidepressant medication targeting innate immune molecules.”
Story Source:
Materials provided by Kobe University. Note: Content may be edited for style and length.
[ad_2]