[ad_1]
Adding functional imaging to structural imaging of patients with ventricular tachycardia (VT) has the potential to improve current VT ablation strategies, according to new research published in the January issue of The Journal of Nuclear Medicine. Iodine-123 metaiodobenzylguanidine (123I-MIBG) SPECT imaging, when combined with cardiac magnetic resonance imaging (MRI), helped to identify specific subsets of heart tissue more prone to arrhythmia, which may allow physicians to achieve improved VT suppression and shorter procedure times.
Ventricular arrhythmias, or abnormal heartbeats originating from the bottom chambers of the heart, are the main cause of sudden cardiac death in the United States and are responsible for up to 300,000 deaths each year. Ablation of ventricular tachycardia is a proven treatment for arrhythmias in patients with a history of heart attacks. Identifying the area of the increased scar tissue that is responsible for the current arrhythmia and possible future arrhythmias has been challenging, with up to 50% of patients suffering a recurrence during the 6 months following the ablation. “The amount of scar tissue can often account for more than half of the left ventricle myocardium,” noted Timm Dickfeld, MD, PhD, FACC, FHRS, director of electrophysiology research at the University of Maryland School of Medicine. “Ablating such a large amount of the myocardium is often not desirable and very time-intensive.”
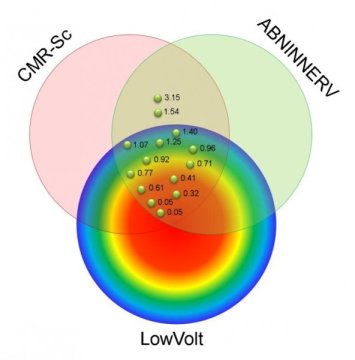
In the study, researchers followed 15 patients with ischemic cardiomyopathy who were scheduled for radiofrequency ablation for drug-refractory VT. Each patient underwent imaging with 123I-MIBG SPECT and cardiac MRI, as well as high-resolution bipolar voltage mapping. These three mapping tools assessed various adaptations found in VT: abnormal innervation, tissue scarring and low-voltage area, respectively. The adaptations were then compared to determine which were present in the affected heart tissue.
Areas with abnormal innervation, cardiac tissue scar and low bipolar voltage were seen in all patients. While approximately 25 percent of patients had abnormalities found by all three mapping tools, researchers found that significant areas of the affected heart tissue showed adaptations only noted by one or two of the tools. The largest of these areas had abnormal innervation only (18.2 percent), cardiac scar tissue and abnormal innervation (14.9 percent), and MRI scar only (14.6 percent). In all cases, the VT site of origin was localized to areas of the tissue with abnormal innervation and MRI scar, identifying an area of abnormal tissue that is likely to be an appropriate target for VT ablation.
“Results from this study show that nuclear medicine can be used to develop novel, cutting-edge strategies for risk stratification and arrhythmia treatment,” said Dickfeld. “The study highlights the importance of close collaboration among the nuclear medicine, radiology and electrophysiology departments, which we are lucky to have at the University of Maryland, to move the field forward. We hope that we can build on the insights provided by this research to develop new treatment algorithms that will result in potentially shorter and more efficient treatment of patients with ventricular arrhythmias.”
The authors of “Ventricular Tachycardia (VT) Substrate Characteristics: Insights from Multimodality Structural and Functional Imaging of the VT Substrate Using Cardiac MRI Scar, 123I-Metaiodobenzylguanidine SPECT Innervation, and Bipolar Voltage” include Hasan Imanli, Kiddy L. Ume, Tamunoinemi Bob-Manuel, Mohammed Abdulghani, Yousra Ghzally, Alejandro Restrepo, Vincent Y. See, Stephen Shorofsky and Timm Dickfeld, Maryland Arrhythmia and Cardiology Imaging Group (MACIG), Baltimore, Maryland, and Division of Cardiology, Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland; Jean Jeudy, Mark F. Smith, Wengen Chen and Vasken Dilsizian, Maryland Arrhythmia and Cardiology Imaging Group (MACIG), Baltimore, Maryland, and Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Maryland, Baltimore, Maryland; Jagat Bandhu Mahat, Division of Cardiology, Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland; and Refael Itah, Biosense Webster, Haifa, Israel.
This study was made available online in June 2018 ahead of final publication in print in January 2019.
[ad_2]