[ad_1]
A cellular protein that interacts with invading viruses appears to help enable the infection process of the Zika virus, according to an international team of researchers who suggest this protein could be a key target in developing new therapies to prevent or treat Zika virus infection.
Scientists first isolated Zika, a member of the Flaviviridae family of viruses — which also includes yellow fever, dengue and West Nile viruses — in 1947 and, until recently, it typically caused only mild symptoms in humans. However, health officials first recorded larger outbreaks of the mosquito-borne virus in 2007, culminating in a large epidemic in the Western Hemisphere in 2015-2016. For the first time, Zika infection also was associated with severe symptoms, including microcephaly in infants infected in the womb, and Guillain-Barre syndrome in adults.
These acute symptoms and the rapid spread of the virus prompted the World Health Organization to declare Zika a public health emergency of international concern and stimulated interest among scientists in the factors governing Zika infection, about which little is known.
In general, flavivirus infection in mammalian cells is mediated by an array of cell surface molecules and attachment cofactors, explained Sujit Pujhari, assistant research professor of entomology, College of Agricultural Sciences, Penn State. Previous work showed that heat shock protein 70 (Hsp70) is one such host molecule for several viruses, including dengue virus, Japanese encephalitis virus and rotavirus.
“In these instances, Hsp70 may act directly as a receptor or indirectly to help attach viruses on the cell surface, where they can interact with specific receptors,” Pujhari said. “Hsp70 also plays a role in controlling viral replication in several virus types, including influenza A and rabies. We hypothesized that Hsp70 might have similar functions in relation to Zika.”
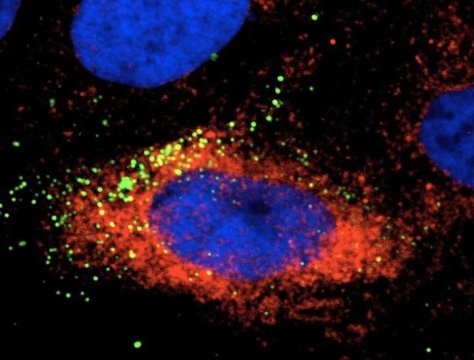
The researchers, who published their findings in Emerging Microbes & Infections, tested this hypothesis by infecting human liver, human nerve and monkey kidney cells with Zika virus. When they used an Hsp70 antibody or a recombinant Hsp70 as a competitor to block the interaction between the protein and the virus, the amount of infectious virus in the cells was reduced, indicating that Hsp70 is involved with Zika virus’s ability to enter cells.
Researchers also found that Hsp70 influences replication of the virus. Their results showed that Zika virus infection induces the expression of Hsp70, and when they treated cells with an Hsp70 inhibitor to reduce production of the protein, it also reduced the production of infectious Zika virus particles. Similarly, when they induced overexpression of Hsp70, production of infectious viral particles was significantly elevated.
In addition, the team found that Zika-infected cells treated with the Hsp70 inhibitor contained increased levels of virus particles compared to the amount of observed extracellular virus. This accumulation of intracellular virus particles indicated that Hsp70 plays a role in the release of mature virus particles from cells.
“These findings show that Hsp70 is an integral host molecule for Zika virus infection of host cells, effecting multiple stages of the infection cycle — virus attachment to cells, viral replication inside cells and virus exit from infected cells,” said Jason Rasgon, professor of entomology and disease epidemiology, Penn State.
“Because there currently is no effective treatment or vaccine for Zika infection, it’s extremely important to develop therapies to reduce infection and severe clinical outcomes, particularly for pregnant women and developing fetuses,” he said. “The multiple critical roles of Hsp70 in the infectious life cycle of Zika virus validate Hsp70 as a potential target for future anti-Zika therapies.”
The research team also included Marco Brustolin and Vanessa Macias, postdoctoral scholars in entomology; Ruth Nissly, research technician and Suresh Kuchipudi, clinical associate professor, both in veterinary and biomedical sciences, Penn State. Also on the project was Masashi Nomura, associate professor, faculty of horticulture, Chiba University, Chiba, Japan. The Penn State Huck Institutes of the Life Sciences and the National Institutes of Health, National Institute of Allergy and Infectious Diseases supported this study.
Story Source:
Materials provided by Penn State. Original written by Chuck Gill. Note: Content may be edited for style and length.
[ad_2]