When animal, insect or human embryos grow in a malnourished environment, their developing nervous systems get first pick of any available nutrients so that new neurons can be made.
In this process, called organ sparing, resources are preferentially delegated to the nervous system at the cost of less important organs or tissues.
New research now shows that developing nervous systems demonstrate this preferential growth even at the level of individual neurons. In a paper published in eLife June 22, “Low FoxO expression in Drosophila somatosensory neurons protects dendrite growth under nutrient restriction,” a team of Cornell researchers discovered the molecular mechanism that helps facilitate organ sparing on this cell-by-cell basis.
“The phenomena we found is similar to the phenomena of the sparing of the brain, but there are very important differences,” said Chun Han, senior author and a Nancy M. and Samuel C. Fleming Associate Professor in the Department of Molecular Biology and Genetics in the College of Agriculture and Life Sciences and in the Weill Institute for Cell and Molecular Biology. “The neurons are protected at the growth level of individual neurons, and they become bigger and bigger by extending their branches.”
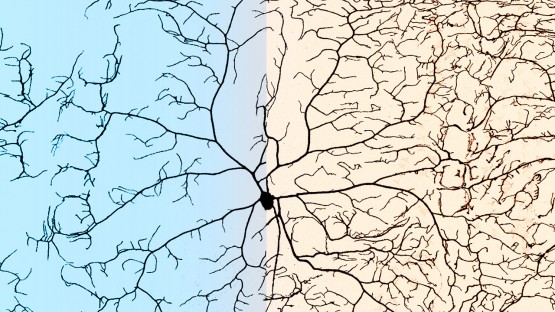
Those branches are called dendrites. They form a system of elaborate arms that extend from neurons’ cellular bodies, and they can receive stimuli from the external environment.
Han and his team wanted to look at how nutrient deficiency affects the dendrite growth of individual neurons, and then examine what cellular sacrifices bodies make so that vital organs, including the brain, continue to develop.
They divided Drosophila (fruit fly) larva into groups receiving either a high- or low-yeast diet, simulating nutrient-rich and nutrient-poor environments. Then they observed how neural cells developed compared to neighboring skin cells on the body wall. They monitored the progress every 24 hours using confocal microscopy that uses lasers to light up fluorescent markers that label individual cells.
“We have very beautiful markers that specifically label these populations of neurons,” Han said. “Every neuron is very clear to us – down to every single branch.”
The researchers observed that the neurons grew at a much higher rate than skin cells in the low-yeast environment. Skin cells grew faster when there was less competition for nutrients. Han and his team learned that this difference is due to a critical gene called FoxO – an important regulator of cellular stress response.
“FoxO is a gene that’s expressed in pretty much most cells of the body,” Han said. “When the cells face low nutrients, FoxO puts a brake on the system and slows cell growth.”
What’s particularly interesting about FoxO is that just because most cells have it, doesn’t mean they all use it at the same time or under the same conditions. Han’s team discovered that even during malnutrition, the Drosophila neurons expressed very little FoxO, whereas the epidermal cells expressed FoxO at much higher levels.
When there are fewer nutrients available, FoxO triggers a response in epidermal cells called autophagy, which tells the cell to self-destruct by consuming itself. However, the limited FoxO expression in neurons preserves individual neural cells and their dendrite growth.
And while humans have more complex systems than Drosophila, Han said that this research helps pave the way for investigating similar phenomenon in humans.
“Our study reveals another layer of nervous system sparing under nutrient deficiency and discovers a novel mechanism by which neurons are protected.” Han said. “These findings may facilitate the development of better approaches to treat problems caused by malnutrition during early development.
Co-authors include Amy Poe, Ph.D. ’18; graduate student Yineng Xu; Christine Zhang ’19; Joyce Lei ’21; Kailyn Li ’17; and David Labib ’20; they conducted research through the Han Lab in the Weill Institute for Cell and Molecular Biology and the Department of Microbiology and Genetics. Poe is currently a postdoctoral researcher at the University of Pennsylvania Perelman School of Medicine; Li is currently in the Doctor of Medicine Program at Weill Cornell Medicine.
This research was supported by a Cornell startup fund and two grants from the National Institutes of Health.
Jana Wiegand is the editorial content manager for the College of Agriculture and Life Sciences.