view more
Credit: RMIT University
New research is helping unlock the mystery of how the brain folds as a baby develops in the womb – a process critical to healthy brain function.
Misfolding of the brain is linked with many neurological conditions including autism, anorexia, epilepsy and schizophrenia.
Premature birth and foetal growth restriction, major risk factors for cerebral palsy, can also affect brain folding and lead to cognitive deficits.
While we know folds are essential to how a healthy human brain works, scientists are only beginning to understand what drives the folding process and why it goes wrong.
A new pre-clinical study by Australian and Swiss researchers has for the first time identified the genes linked with the development of the two types of brain folds – inward and outward – in the grey matter of the brain.
Using animal models that closely resemble human brain development, the study found differences in both genetic expression and neuron shape during the folding process.
Co-chief investigator Associate Professor Mary Tolcos said there were currently no therapies for the prevention or treatment of brain misfolding and no early test to detect problems before folding begins.
“We know folding happens in the second and third trimester of pregnancy and that all human brains fold along largely the same patterns,” said Tolcos, an Australian Research Council Future Fellow at RMIT.
“This suggests the process is heavily controlled by our genes but we’re only starting to understand how – it’s like having an intricately folded piece of origami that’s missing the instructions.
“Our study is a critical step towards fully understanding those genetic instructions, by pinpointing which genes are linked with fold development.
“The next step is to determine the precise role these genes play in the process, so we can work towards identifying potential therapeutic targets and develop interventions to prevent and fix misfolding in the brain.”
Folding fundamentals
Previous brain folding studies have focused on white matter or looked at animals with smooth brains rather than folded ones, but have largely overlooked grey matter.
Grey matter is made up of neuron bodies and their connecting arms, while white matter is composed of the neurons’ long nerve fibres and their protective layer of fat.
While the science of folding is still unclear, the latest evidence suggests grey matter in the developing brain expands faster than white matter, creating mechanical instability that leads to brain folding.
But the resulting “hill” and “valley” folds are not random – they follow a similar pattern in all folded brains of the same species.
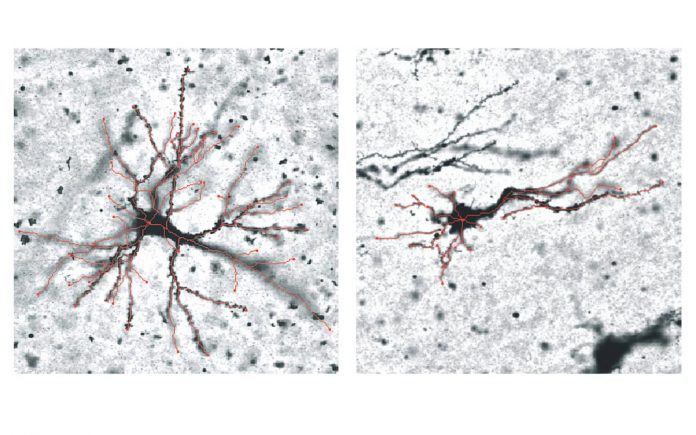
The new study focused on sheep brains, which fold in the womb like the human brain.
Researchers investigated the genetic and microstructural differences in future grey matter, the cortical plate, in the parts of the brain just beneath the “hills” and “valleys”.
The areas were analysed at three points of development – when the brain was smooth, semi-folded and fully folded.
Postdoctoral researcher Dr Sebastian Quezada Rojas said it was the first study to look at the genetics of folding of the future grey matter, as the folding process develops over time.
“Understanding the changes in the cortical plate during brain folding is paramount since this region changes so dramatically during this process,” he said.
“We found some genes have higher expression in regions that fold outward and lower expression in regions that fold inwards. Other genes reverse this pattern.
“Together, these genetic expression patterns might explain why the cortical folding pattern is so consistent between individuals of the same species.”
These genetic differences are also correlated with changes in grey matter neurons, with the study finding variations in the number of arms – or dendrites – that neurons grow in these regions during the folding process.
“We believe the regions that fold outward and inward are programmed to behave differently, and the shape of the neurons affects the way these areas fold. Untangling these connections will be focus of the next stage of this research,” Quezada Rojas said.
###
The research, supported with funding from the Australian Research Council (ARC) and National Health and Medical Research Council (NHMRC), was co-led by Professor David Walker, Head of the Neurodevelopment in Health and Disease research program in the School of Health and Biomedical Sciences at RMIT.
‘Genetic and microstructural differences in the cortical plate of gyri and sulci during gyrification in fetal sheep’, with collaborators from the Federal Institute of Technology of Lausanne, Monash University and University of Geneva, is published in Cerebral Cortex (DOI: 10.1093/cercor/bhaa171).
TDnews