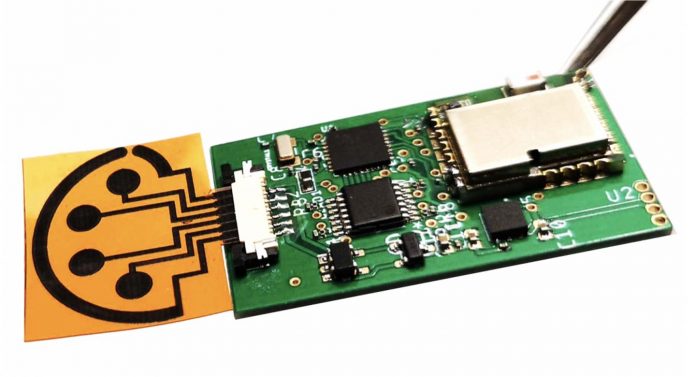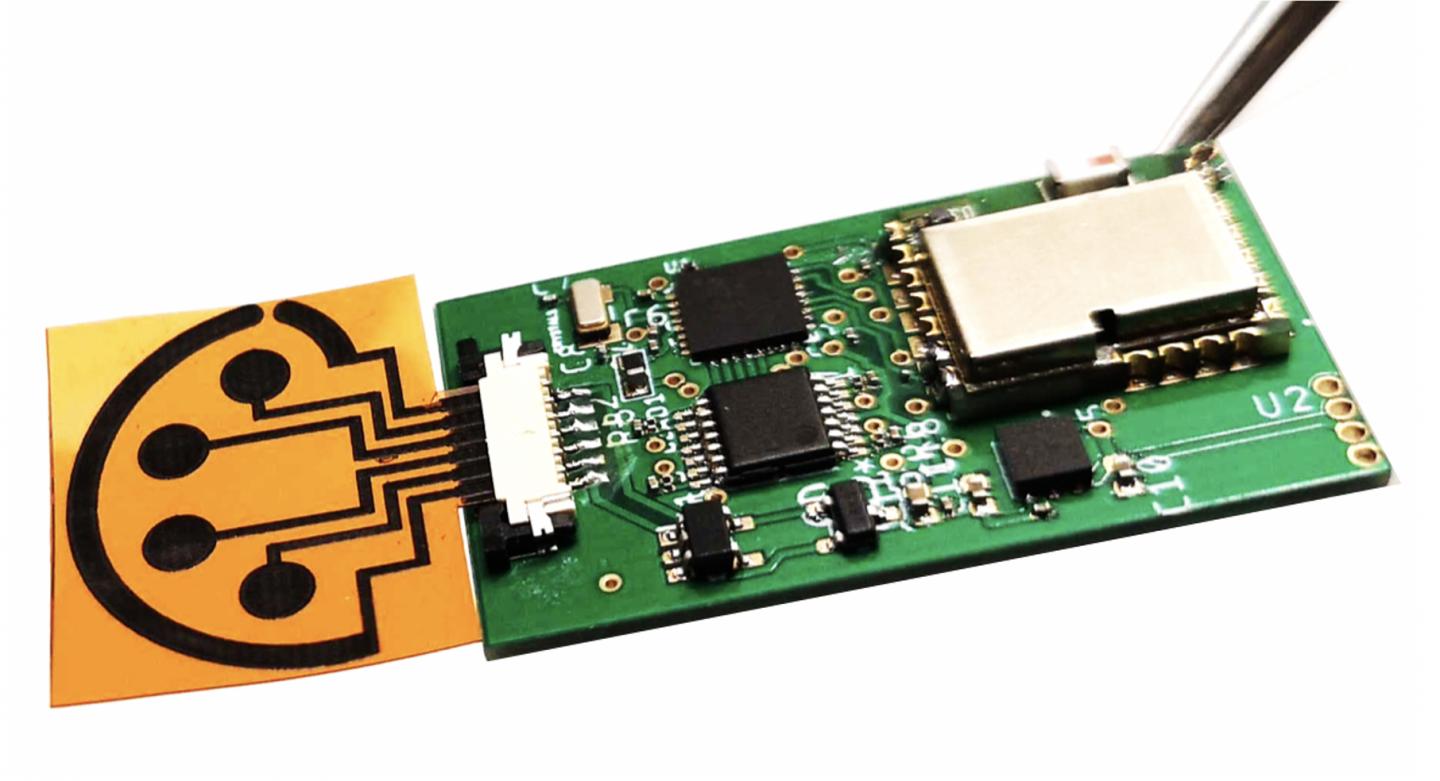
Photo: When attached to supporting electronics, the sensor can wirelessly transmit data to the user’s cell phone through Bluetooth.
view more
Credit Image: Caltech
One feature of the COVID-19 virus that makes it so difficult to contain is that it can be easily spread to others by a person who has yet to show any signs of infection. The carrier of the virus might feel perfectly well and go about their daily business–taking the virus with them to work, to the home of a family member, or to public gatherings.
A crucial part of the global effort to stem the spread of the pandemic, therefore, is the development of tests that can rapidly identify infections in people who are not yet symptomatic.
Now, Caltech researchers have developed a new type of multiplexed test (a test that combines multiple kinds of data) with a low-cost sensor that may enable the at-home diagnosis of a COVID infection through rapid analysis of small volumes of saliva or blood, without the involvement of a medical professional, in less than 10 minutes.
The research was conducted in the lab of Wei Gao, assistant professor in the Andrew and Peggy Cherng department of medical engineering.. Previously, Gao and his team have developed wireless sensors that can monitor conditions such as gout, as well as stress levels, through the detection of extremely low levels of specific compounds in blood, saliva, or sweat.
Gao’s sensors are made of graphene, a sheet-like form of carbon. A plastic sheet etched with a laser generates a 3D graphene structure with tiny pores. Those pores create a large amount of surface area on the sensor, which makes it sensitive enough to detect, with high accuracy, compounds that are only present in very small amounts. In this sensor, the graphene structures are coupled with antibodies, immune system molecules that are sensitive to specific proteins, like those on the surface of a COVID virus, for example.
Previous versions of the sensor were impregnated with antibodies for the hormone cortisol, which is associated with stress, and uric acid, which at high concentrations causes gout. The new version of the sensor, which Gao has named SARS-CoV-2 RapidPlex, contains antibodies and proteins that allow it to detect the presence of the virus itself; antibodies created by the body to fight the virus; and chemical markers of inflammation, which indicate the severity of the COVID-19 infection.
“This is the only telemedicine platform I’ve seen that can give information about the infection in three types of data with a single sensor,” Gao says. “In as little as a few minutes, we can simultaneously check these levels, so we get a full picture about the infection, including early infection, immunity, and severity.”
Established COVID-testing technologies usually take hours or even days to produce results. Those technologies also require expensive, complicated equipment, whereas Gao’s system is simple and compact.
So far, the device has been tested only in the lab with a small number of blood and saliva samples obtained for medical research purposes from individuals who have tested positive or negative for COVID-19. Though preliminary results indicate that the sensor is highly accurate, a larger-scale test with real-world patients rather than laboratory samples must be performed, Gao cautions, to definitively determine its accuracy.
With the pilot study now completed, Gao next plans to test how long the sensors last with regular use, and to begin testing them with hospitalized COVID-19 patients. Following in-hospital testing, he would like to study the suitability of the tests for in-home use. Following testing, the device will need to receive regulatory approval before it is available for widespread use at home.
“Our ultimate aim really is home use,” he says. “In the following year, we plan to mail them to high-risk individuals for at-home testing. And in the future, this platform could be modified for other types of infectious disease testing at home.”
###
The paper describing the research, titled, “SARS-CoV-2 RapidPlex: A Graphene-based Multiplexed Telemedicine Platform for Rapid and Low-Cost COVID-19 Diagnosis and Monitoring,” has been published online and will appear in the December issue of the journal Matter. Co-authors are former postdoctoral scholar in medical engineering Rebeca M. Torrente-Rodríguez, medical engineering graduate students Heather Lukas, Jiaobing Tu (MS ’20), Jihong Min (MS ’19), Yiran Yang (MS ’18), and Changhao Xu (MS ’20); and Harry B. Rossiter of the Harbor-UCLA Medical Center.
Funding for the research was provided by the National Institutes of Health; the Tobacco-Related Disease Research Program, a California state agency focused on reducing tobacco use; the Merkin Institute for Translational Research; and the Translational Research Institute for Space Health.
TDnews (tunisiesoir.com)