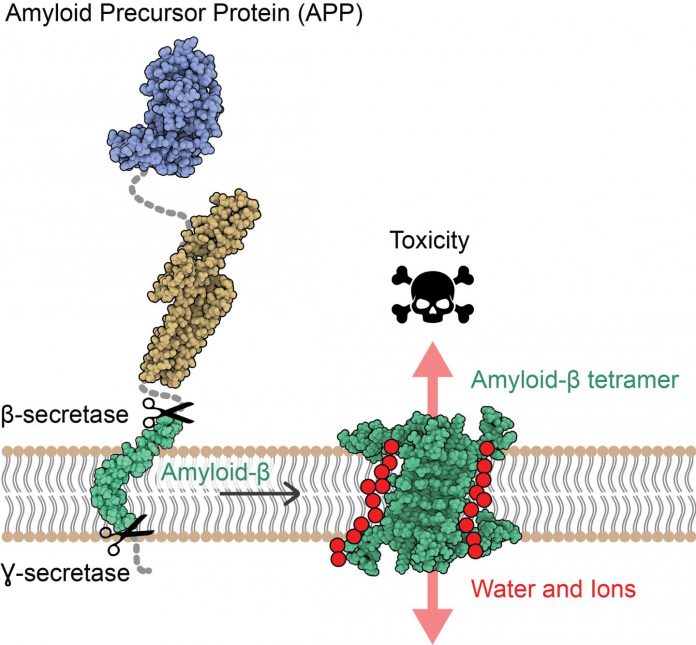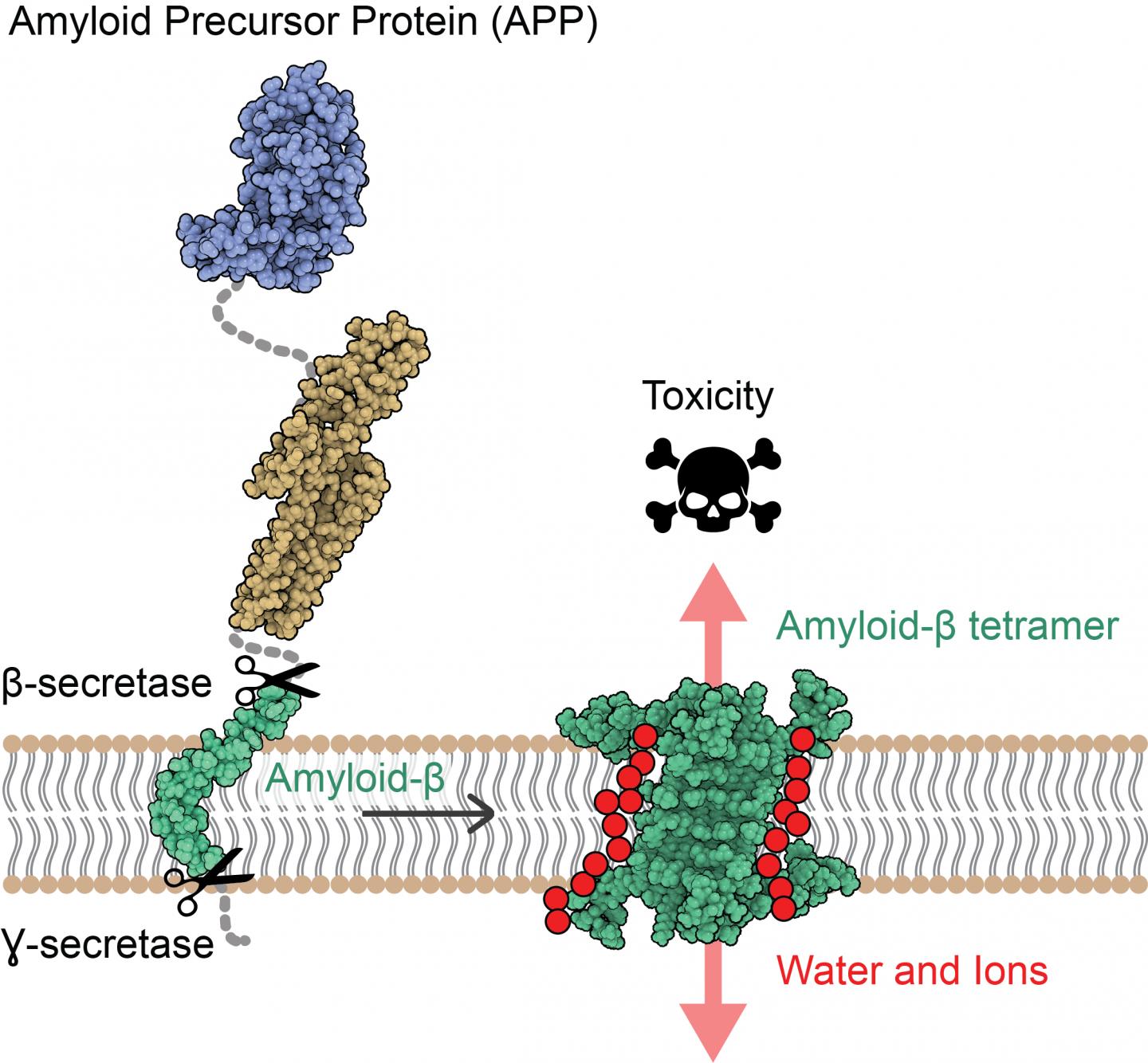
IMAGE: The amyloid precursor protein (APP) is inserted into the cell membrane of neurons. After sequential cleavage by β- and ?-secretases, the Aβ protein (in green) is released.
The membrane oligomers are…
view more
Credit: Benjamin Bardiaux.
The brains of millions of people suffering from Alzheimer´s disease (AD) are slowly and inescapably being depleted of neurons, which leads to the characteristic loss of memory and cognitive function associated with this condition. However, the cause of neuronal death is still unknown. The treatments available are aimed at slowing down the development of dementia and only help to improve quality of life for short periods. Therefore, treatments to actually cure AD are an unmet medical need.
Researchers led by Natalia Carulla, IRB Barcelona Alumni, former group leader at the Institut Européen de Chimie et Biologie (IECB) in Bordeaux, and currently project manager at Grup CIEF have revealed for the first time the atomic structure of amyloid-beta (Aβ) protein assemblies. The knowledge of this structure reveals a new mechanism of toxicity for these assemblies, with the capacity to disrupt the neuronal membrane, allowing water and ions to pass through it and causing the death of these cells. Several studies have proposed that the interaction of the Aβ protein with the neuronal membrane is responsible for the neuronal death observed in AD. However, the Aβ protein is a difficult therapeutic target because it is “sticky” and self-assembles, adopting distinct shapes and sizes.
“Knowing the features that characterise these protein ensembles, such as the number of molecules that make them and the shape they adopt, is crucial to design effective therapeutic strategies that target the forms of Aβ ensembles responsible for the neurotoxicity in AD,” Carulla explains.
An in vitro approach to ensure stable Aβ forms
To tackle the instability of the different conformations, the team first studied the Aβ protein in vitro–in simplified model systems that mimic the neuronal membrane–to develop conditions to prepare stable Aβ forms of uniform composition and shape. Once the different compositions had been identified, they studied their structure and mode of neurotoxicity, establishing a 3D arrangement of all the atoms making up the Aβ ensemble.
“Our study suggests that some Aβ associations can perforate the membrane of neurons, alter their osmotic equilibrium, and consequently trigger their death,” say Sonia Ciudad and Eduard Puig, first authors of the paper. Ciudad is an IRB Barcelona Alumni, currently R&D Scientist at Biokit, a Werfen Company; while Puig is now a postdoctoral fellow in Research Unit on Asymmetric Synthesis at IRB Barcelona.
Targeting membrane pores to avoid neurotoxicity
This study has highlighted two Aβ protein ensembles, one formed by four Aβ proteins and the other by eight, whose arrangement has the capacity to disrupt cell membrane, proposing them as candidates for causing neurodegeneration in AD.
Further work should focus on approaches to prevent the formation of this ensemble-thus preventing membrane disruption. Currently, the drug discovery pipeline in this field does not include any drug targeting membrane-associated Aβ assemblies, so this finding could make a significant breakthrough in the treatment of AD.
###
This work was funded by MINECO (SAF2015-68789), the Fundació La Marató de TV3 (20140730), the Fondation Recherche Médicale (AJE20151234751), and the Counseil Régional d’Aquitaine Limousin Poitou-Charentes (1R30117-00007559). It was performed in collaboration with Vladislav Orekhov’s laboratory at the Swedish NMR Centre, University of Gothenburg, Benjamin Bardiaux at the Institut Pasteur, Stéphane Chaignepain at CBMN, Sarah Cianferani’s laboratory at the Université de Strasbourg, Giovanni Maglia’s laboratory at the Groningen Biomolecular Sciences & Biotechnology Institute, and Emad Tajkhorshid’s laboratory at the NIH Center for Macromolecular Modeling and Bioinformatics.
TDnews