[ad_1]
The parasitic disease schistosomiasis is one of the developing world’s worst public health scourges, affecting hundreds of millions of people, yet only a single, limited treatment exists to combat the disease.
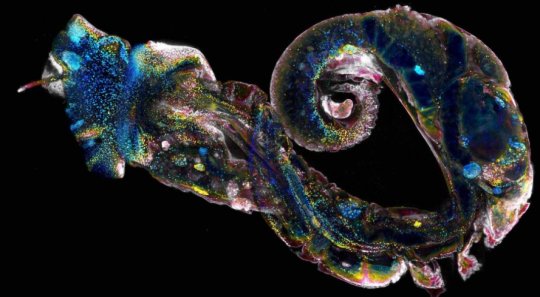
Researchers at the Morgridge Institute for Research are searching for potential new targets by probing the cellular and developmental biology of its source, the parasitic flatworm Schistosoma.
The team’s newest work, published today in the journal eLife, sheds light on essential stages in the life cycle of this blood fluke. They characterized several different types of stem cells that govern the parasite’s complex life cycle and also identified a gene associated with the earliest development of the germline, from which gametes form.
“Understanding how these stem cells drive the development of each life-cycle stage may ultimately help prevent disease transmission,” says senior author Phillip Newmark, a Howard Hughes Medical Institute and Morgridge investigator and professor of integrative biology at the University of Wisconsin-Madison.
More than 250 million people, mostly in Africa and Asia, have schistosomiasis. The World Health Organization classifies it as the deadliest neglected tropical disease, killing an estimated 280,000 people each year. Children with the disease are often ravaged by anemia, malnutrition and pervasive learning disabilities.
The drug Praziquantel is the primary form of treatment. The drug is largely effective in killing the adult worms in humans, but not in the parasite’s other life cycle stages, leaving people exposed to continual reinfection.
Schistosomes have a complicated life cycle, switching through many different body plans as they move from snails to water to humans. The cycle begins in tainted freshwater lakes and ponds, where parasite eggs released from human waste hatch into tiny creatures whose sole task is to infect a specific type of snail.
Within the snail host, the parasite produces massive numbers of offspring called cercariae. These fast-swimming, fork-tailed organisms are released into the water, from which they burrow through human skin and cause infection.
After penetrating host skin, the parasites must migrate into the blood vessels and find their way to the major vein that supplies the liver. During this journey, the parasites reorganize their tissues, and upon reaching the liver, begin developing reproductive organs, pair with a mate, and grow into mature adults.
The team examined the poorly understood early stages after infection through an ingenious experiment designed by co-author Jayhun “Jay” Lee, a Morgridge Postdoctoral Fellow in the Newmark Lab. He mimicked infection in a culture dish by enabling cercariae to penetrate through a portion of mouse skin into a medium on the other side. This strategy allowed him to examine when and which cells first begin to divide after infection.
“We don’t get that many ah-ha moments in our lives as scientists,” Newmark says. “This was one of them.”
During the initial 22-36 hours of infection, they observed five distinct cells proliferating; these same stem cells were packed into the cercariae during development inside the snail. The cells give rise to the adult stem cells, initiating development of the parasite into the adult worm. From there, they identified a subset of stem cells associated with development of the reproductive system.
“We’re really excited about this because it opens up a number of important research directions,” Newmark says. “The drug used to fight schistosomes does not work on this stage of infection. Understanding what’s happening in this early period after infection is critical, because it’s also a time when the parasites should be most vulnerable.”
Lee says the next research step will be to follow these five stem cells as they continue to differentiate and form tissues. “We want to use this as a road map to figure out what the cells are doing,” he says.
The Newmark Lab’s primary focus has been on exploring regeneration in planarians — remarkable flatworms that can regenerate from the tiniest body fragments. They started work on schistosomes, “evil cousins” of planarians, in 2009 and applied decades of planarian biology to better understand their parasitic relatives.
It’s a case where model organism research may help provide answers for a human health tragedy. “This provides another example of how curiosity-driven basic research can lead to unanticipated outcomes and why it is important to support such work,” Newmark says.
[ad_2]