[ad_1]
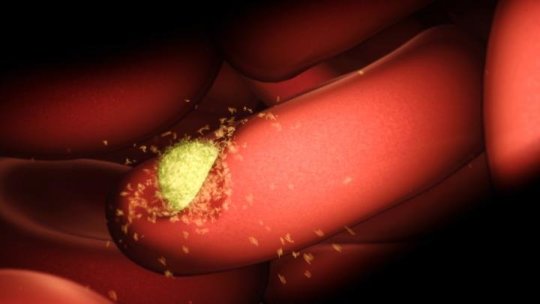
Melbourne scientists have taken a significant step toward developing a new vaccine for malaria, revealing for the first time an ‘atomic-scale’ blueprint of how the parasite invades human cells.
Using the Nobel Prize-winning technology cryo-EM (cryo-electron microscopy), the researchers mapped the previously hidden first contact between Plasmodium vivax malaria parasites and young red blood cells they invade to begin the parasites’ spread throughout the body. The discovery was published today in Nature.
Associate Professor Wai-Hong Tham and Dr Jakub Gruszczyk from Melbourne’s Walter and Eliza Hall Institute — in collaboration with Dr Rick Huang and Dr Zhiheng Yu at the Howard Hughes Medical Institute (US) — solved the mystery of the molecular machinery the parasite uses to latch on to red blood cells.
This essential step in the malaria lifecycle is the beginning of the classical symptoms associated with malaria — fever, chills, malaise, diarrhea and vomiting — which can last weeks or even longer.
Cryo-EM provides vaccine key
Earlier this year, the team discovered P. vivax parasites use the human transferrin receptor to gain access to red blood cells, a study they published in Science. Now, with the aid of revolutionary cryo-EM technology, Associate Professor Tham said the team was able to overcome previous technical challenges to visualise the interaction at an atomic level.
“We’ve now mapped, down to the atomic level, exactly how the parasite interacts with the human transferrin receptor,” Associate Professor Tham said.
“This is critical for taking our original finding to the next stage — developing potential new antimalarial drugs and vaccines. Cryo-EM is really opening doors for researchers to visualise structures that were previously too large and complex to ‘solve’ before.”
P. vivax is the most widespread malaria parasite worldwide, and the predominant cause of malaria in the vast majority of countries outside Africa. Because of its propensity to ‘hide’ undetected by the immune system in a person’s liver, it is also the number one parasite responsible for recurrent malaria infections.
Guided by the 3D map, Associate Professor Tham said the team was able to tease out the precise details of the parasite-host interaction, identifying its most vulnerable spots.
“It’s basically a design challenge. P. vivax parasites are incredibly diverse — which is challenging for vaccine development. We have now identified the molecular machinery that would be the best target for an antimalarial vaccine effective against the widest range of P. vivax parasites,” she said.
“With this unprecedented level of detail, we can now begin to design new therapies that specifically target and disrupt the parasite’s invasion machinery, preventing malaria parasites from hijacking human red blood cells to spread through the blood and, ultimately, be transmitted to others.”
Exploiting weak spots
Dr Gruszczyk said the team also ‘solved’ how antimalarial antibodies bind to and block P. vivax parasites to stop them from invading red blood cells, using X-ray crystallography facilities at the Australian Synchrotron.
“With this crystal map, we have identified additional ‘weak spots’ that could be exploited as therapeutic targets. The information allows us to go back to the parasite and pull out the part of the protein that will make the best possible vaccine,” Dr Gruszczyk said.
The research was supported by the Australian Research Council, Speedy Innovation Grant, National Health and Medical Research Council, Howard Hughes Medical Institute, Wellcome Trust, Drakensberg Trust and the Victorian Government.
[ad_2]