[ad_1]
The 2018 Nobel Prize in Chemistry went to three scientists who developed the method that forever changed protein engineering: directed evolution. Mimicking natural evolution, directed evolution guides the synthesis of proteins with improved or new functions.
First, the original protein is mutated to create a collection of mutant protein variants. The protein variants that show improved or more desirable functions are selected. These selected proteins are then once more mutated to create another collection of protein variants for another round of selection. This cycle is repeated until a final, mutated protein is evolved with optimized performance compared to the original protein.
Now, scientists from the lab of Ardemis Boghossian at EPFL, have been able to use directed evolution to build not proteins, but synthetic nanoparticles. These nanoparticles are used as optical biosensors — tiny devices that use light to detect biological molecules in air, water, or blood. Optical biosensors are widely used in biological research, drug development, and medical diagnostics, such as real-time monitoring of insulin and glucose in diabetics.
“The beauty of directed evolution is that we can engineer a protein without even knowing how its structure is related to its function,” says Boghossian. “And we don’t even have this information for the vast, vast majority of proteins.”
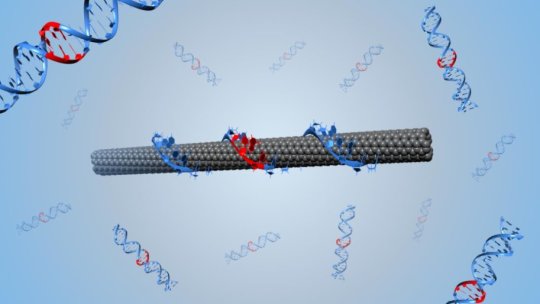
Her group used directed evolution to modify the optoelectronic properties of DNA-wrapped single-walled carbon nanotubes (or, DNA-SWCNTs, as they are abbreviated), which are nano-sized tubes of carbon atoms that resemble rolled up sheets of graphene covered by DNA. When they detect their target, the DNA-SWCNTs emit an optical signal that can penetrate through complex biological fluids, like blood or urine.
General principle of the directed evolution approach applied to the nanoparticle DNA-SWCNT complexes. The starting complex is a DNA-SWCNT with a dim optical signal. This is evolved through directed evolution: (1) random mutation of the DNA sequence; (2) wrapping of the SWCNTs with the DNA and screening of the complex’s optical signal; (3) selection of the DNA-SWCNT complexes exhibiting an improved optical signal. After several cycles of evolution, we can evolve DNA-SWCNT complexes that show enhanced optical behavior. Credit: Benjamin Lambert (EPFL)
Using a directed evolution approach, Boghossian’s team was able to engineer new DNA-SWCNTs with optical signals that are increased by up to 56% — and they did it over only two evolution cycles.
“The majority of researchers in this field just screen large libraries of different materials in hopes of finding one with the properties they are looking for,” says Boghossian. “In optical nanosensors, we try to improve properties like selectivity, brightness, and sensitivity. By applying directed evolution, we provide researchers with a guided approach to engineering these nanosensors.”
The study shows that what is essentially a bioengineering technique can be used to more rationally tune the optoelectronic properties of certain nanomaterials. Boghossian explains: “Fields like materials science and physics are mostly preoccupied with defining material structure-function relationships, making materials that lack this information difficult to engineer. But this is a problem that nature solved billions of years ago — and, in recent decades, biologists have tackled it as well. I think our study shows that as materials scientists and physicists, we can still learn a few pragmatic lessons from biologists.”
Story Source:
Materials provided by Ecole Polytechnique Fédérale de Lausanne. Note: Content may be edited for style and length.
[ad_2]