[ad_1]
In organic chemistry, discovering new reactions is one thing, but to carry them off efficiently is quite another. Carbon-carbon bond-forming is at the heart of organic synthesis, allowing us to stitch together diverse functional groups into an endless array of useful compounds. Now, Kanazawa University researchers have neatly streamlined one of the most important of those reactions.
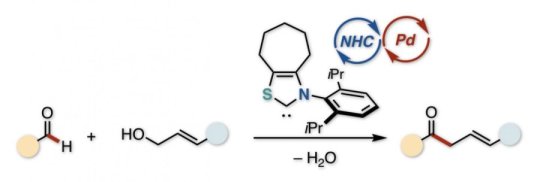
Unsaturated ketones contain a C=O group and a C=C bond. If those two are separated by a CH2 group, the ketone is of the “beta-gamma” type. Logically enough, they can be made by reacting a C=O carbonyl with an allylic (C=C-containing) alcohol. However, in the usual route, these must be pre-activated with a metal and an organic leaving group, respectively, which is laborious and costly.
“React an aldehyde directly with an alcohol, and you do away with the expensive pre-activation,” says Hirohisa Ohmiya, leading author of the study, published in Chemistry: A European Journal. “As an added benefit, the by-product is merely water, formed by expelling an H atom and an OH group. Unfortunately, the carbon atoms that we need to react normally repel each other, because they are both positively charged.”
However, as the study shows, the dehydrative (water-releasing) reaction can be stimulated by a classic trick in the chemist’s arsenal: umpolung, or reversing the polarity. The normally electrophilic (positive) C=O carbon is transformed via catalysis. Primed by the electron density donated from an N-heterocyclic carbene (NHC) catalyst, the carbonyl becomes a negatively charged nucleophile, ready to react with the electrophilic alcohol.
Both aldehydes and allylic alcohols are easily available, and the Kanazawa team synthesized a diversity of products in good yield. Many biological compounds are beta-gamma unsaturated ketones — for example, the team produced a steroid derivative through selective reaction of the desired steroid C=O group. This class of ketones is also an important stop on the route to larger molecules.
While the NHC catalyst prepares the carbonyl substrate for the reaction, a second catalyst is needed to activate the alcohol. This phosphine-bound palladium complex, generated in situ during the reaction, forms a cation that undergoes allylation with the negatively charged NHC/carbonyl anion. The combination of catalysts is a neat example of synergistic cooperation during a reaction.
“This will undoubtedly make beta-gamma unsaturated ketones much more accessible,” says lead author Hirohisa Ohmiya. “Chemists have a responsibility to the environment like everyone else, so the lack of by-products and the economical nature of the reaction will be major assets when scaled-up to industry. Our next challenge is to devise a chirally selective version for asymmetric synthesis.”
Story Source:
Materials provided by Kanazawa University. Note: Content may be edited for style and length.
[ad_2]