[ad_1]
A team of scientists from the New York Stem Cell Foundation (NYSCF) Research Institute reported Friday in Stem Cell Research and Therapy that they have made valuable progress toward creating clinical-grade cells for treatment of bone disease and injury. In their study, the team identified two types of growth media that could support effective expansion of mesenchymal progenitor (MP) cells from stem cells in a clinically compatible, Good Manufacturing Practice (GMP) setting. GMP guidelines require that cells to be used as therapies are created without the use of animal-derived substances.
“NYSCF is committed to bringing effective cellular therapies to patients in need,” says NYSCF CEO Susan L. Solomon. “To establish these therapies, it is essential to produce high-quality cells that meet safety requirements for clinical use, which is a step that this research is helping us achieve.”
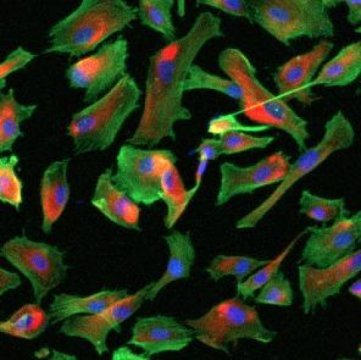
MP cells are important because they resemble mesenchymal stem cells (MSCs). MSCs can go on to form a variety of cell types, including bone cells, cartilage cells, muscle cells, and fat cells, and can modulate the behavior of many other types of cell types in the body. They are a frequent target for cell therapies in which healthy cells are introduced into the body to treat diseases or reconstruct tissues and organs. However, MSCs are often scarce and do not expand well enough to provide the amount of cells needed for an effective therapy. MP cells, on the other hand, can be produced in large numbers for each patient when generated from induced pluripotent stem cells (iPSCs), and therefore hold extraordinary promise for the treatment of blood, heart, and immune diseases as well as repair of damaged bone and cartilage.
“MP cells have been derived from iPSCs before, but never in a growth medium that does not contain animal-derived compounds,” says NYSCF — Ralph Lauren Senior Investigator Giuseppe Maria de Peppo, PhD, who led the study. “We are glad to see that MP cells grown in GMP-compliant media showed the same biological and functional properties as those grown in research-grade media that contains animal products. The results will help us plan for movement of these cells out of the lab and into the clinic.”
To test his question, the researchers compared MP cells grown in a medium supplemented with fetal bovine serum, a product derived from cows, to MP cells grown in two different media without animal products (referred to as “xeno-free”) — one supplemented with human platelet lysates and one commercial high-performance GMP medium (AllegroTM Unison Medium). The team found that while MP cells grown in the xeno-free and GMP media showed slightly different cell morphology, expansion potential, gene expression, and cytokine profile than those grown in the medium containing fetal bovine serum, the cells were healthy and functional in these new conditions. Collectively, the results show promise for the eventual application of MP cells in cellular therapies.
Story Source:
Materials provided by New York Stem Cell Foundation. Note: Content may be edited for style and length.
[ad_2]